
Heat transfers from an area of _temperature to an area of _ temperature.It is observed that during the change of state. You must be aware that water boils at a temperature of 100 degrees Celsius, that is, 373 Kelvin. The temperature at which the liquid starts boiling at the atmospheric pressure is called its boiling point.
:max_bytes(150000):strip_icc()/143058836-56a12f0a5f9b58b7d0bcdab4.jpg)
The average heat transfer co-efficient for laminar film condensation on vertical surface is inversely proportional to (where, ΔT = Temperature drop across condensate film ) At this temperature, the liquid starts changing into vapors, that is, to the gaseous state.With the increase of temperature, the Col-burn jH factor.Which of the following is not used as a medium for high temperature heating ?.The actual temperature drop across the heating surface in an evaporator depends on the.The log mean temperature difference in ☌ is approximately If you increase the temperature from 293 K to 303 K (20☌ to 30☌), you will increase the collision frequency by a factor of: Thats an increase of 1.7 for a 10° rise. Cold oil (0.05 m3/min) of density 800 kg/m3 and specific heat of 2 kJ/kg.K enters at 20☌. Hot water (0.01 m3 /min) enters the tube side of a counter current shell and tube heat exchanger at 80☌ and leaves at 50☌. Transcribed image text: Question 8 When beat is added absorbed, the temperature of matter increases and the particles.

Neglecting the heat capacity of agitator, the temperature of water (in ☌) is The acceleration due to gravity is 9.8 ms-2. It is stirred by an agitator, which is made to turn by a slowly falling body weighing 40 kg through a height of 4 m.
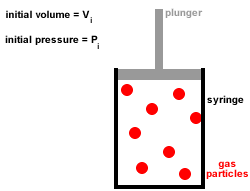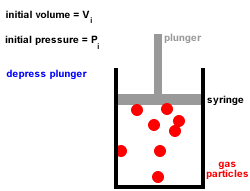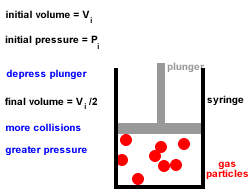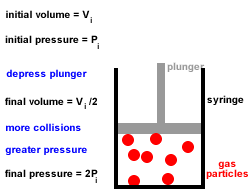
In Joule's experiment, an insulated container contains 20 kg of water initially at 25☌. With increase in temperature, the thermal conductivity of non-metallic amorphous solids. Rate of cooling by big ball as compared to smaller one will be in the ratio of Two balls of same material and finish have their diameters in the ratio of 2: 1 and both are heated to same temperature and allowed to cool by radiation. Which of the following property of air does not increase with rise in temperature?. Thermal conductivity of water _ with rise in temperature. This statement is known as Stefan Boltzmann law. The total radiation from a black body per second per unit area is _ fourth power of the absolute temperature. Temperature of steam at around 540☌ can be measured by. Heat conducted through per unit area and unit thick face per unit time when temperature difference between opposite faces is unity, is called. What is the energy of the movement of particles in a substance?. The process of heat transfer from one particle of the fluid to another by the actual movement of the fluid particles caused by some mechanical means, is known as. When heat is transferred from one particle of hot body to another by actual motion of the heated particles, it is referred to as heat transfer by. The temperature of the inside wall is - 5☌. The heat flux (from outside to inside) across an insulating wall with thermal conductivity, K= 0.04 W/m.°K and thickness 0.16m is 10 W/m2. If an object has particles that are moving very quickly, the object 's temperature is probably _. Give some of the arguments in favour of condensing boilers compared to older non-condensing boilers. Waste water vapour produced when the water is heated in the boiler is used to preheat the cold water entering the boiler. Modern condensing central heating boilers take advantage of the energy that is released when water condenses. Jostling about and colliding increases until. Sample question 3 - Higher QuestionĬentral heating boilers burn gas and use the energy released to heat water. As the temperature of a liquid is increased, the particles gain more energy and move faster and faster. It may be helpful to plan which key words are going to be used and use these to help structure the answer, eg temperature, kinetic energy, speed, collisions, force. Īnswers should include scientific key words. This means that a greater force is applied to the walls of the container and so the pressure increases. Because the particles are moving faster they collide more frequently with the walls of the container. The speed of the particles increases with kinetic energy. Increasing the temperature also increases the kinetic energy of the gas particles.



:max_bytes(150000):strip_icc()/143058836-56a12f0a5f9b58b7d0bcdab4.jpg)



 0 kommentar(er)
0 kommentar(er)
